Materials Science and Electrochemical Engineering for Energy Storage
Our goals are to develop sustainable materials/technologies to produce advanced battery technology with higher energy density, better safety, lower cost, faster charging capability, wider temperature operation range, and longer cycle and calendar lifetime.
Research Areas
Novel Li-ion and Na-ion electrode materials with earth-crust abundant elements
Achieving a zero-carbon transition will require meeting global energy demands with renewable sources of energy. Due to the intermittent nature of many renewable sources, achieving significant levels of integration will demand utility-scale energy storage systems. Li-ion batteries have dominated the market. However, rapidly growing demands in many technology sectors (e.g. electric vehicles, mobile electronics) aggravates the supply chain issues of critical elements, especially lithium, cobalt, nickel. This presents an urgent need of developing battery electrode materials with earth abundant elements (e.g. sodium, manganese) using sustainable technologies (e.g. dry-method).
Image credit: EuChemS/CC BY-ND
Safety Studies of Li-ion and Na-ion batteries
Accelerating Rate Calorimetry (ARC) is used as the major method to study the reactions between charged electrode materials and electrolytes at elevated temperature 1,2. This is a significant step to leverage the safety performance of novel electrode or electrolyte materials before scaling up.
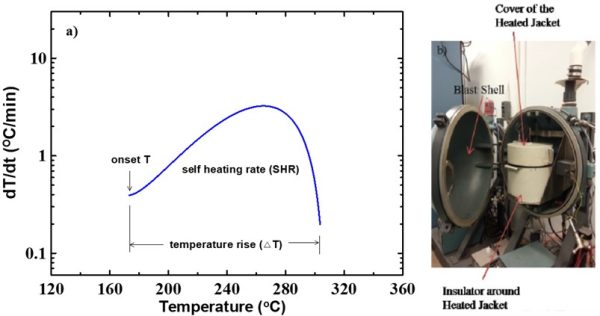
- “A Systematic Study on the Reactivity of Different Grades of Charged Li[NixMnyCoz]O2 with Electrolyte at Elevated Temperatures using Accelerating Rate Calorimetry.” Lin Ma, M. Nie, Jian Xia and J. R. Dahn. J. Power Sources. 2016, 327, 145-150.
- “The Impact of Vinylene Carbonate, Fluoroethylene Carbonate and Vinyl Ethylene Carbonate Electrolyte Additives on Electrode/electrolyte Reactivity Studied using Accelerating Rate Calorimetry.” L. Ma, J. Xia, X. Xia, J. R. Dahn. J. Electrochem. Soc. 2014, 161, A1495-A1498.
Battery Failure Mechanisms Analysis
Rechargeable batteries will eventually fail (as shown in a). A combination of advanced techniques (as shown in b-e) 1-3 is necessary to fundamentally understand the failure mechanisms so that we can develop novel materials to improve cell performance within a short time range.
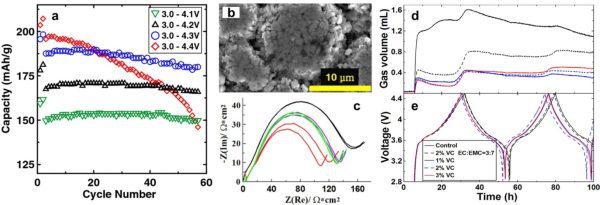
- “A Guide to Ethylene Carbonate-free Electrolyte Making for Li-ion Cells.” Lin Ma, S. L. Glazier, R. Petibon, Jian Xia, Jeremy M. Peters, Q. Liu, J. Allen, R. N. C. Doig and J. R. Dahn. J. Electrochem. Soc., 2017, 164, A5008-A5018.
- “Study of the Failure Mechanisms of LiNi0.8Mn0.1Co0.1O2 Cathode Material for Lithium Ion Batteries.” Jing Li, Laura E. Downie, L. Ma, Wenda Qiu and J. R. Dahn. J. Electrochem. Soc. 2015, 162, A1401-A1408.
- “Sulfolane-Based Electrolyte for High Voltage Li(Ni0.42Mn0.42Co0.16 )O2 (NMC442)/Graphite Pouch Cells.” J. Xia, Julian Self, L. Ma and J. R. Dahn. J. Electrochem. Soc. 2015, 162, A1424-A1431.
Residential Faculty
Lin Ma