Materials Science and Energy Storage
Our goals are to develop sustainable materials/technologies to produce advanced battery technology with higher energy density, better safety, lower cost, faster charging capability, wider temperature operation range, and longer cycle and calendar lifetime.
Research Areas
Novel electrode materials with earth-crust abundant elements
Achieving a zero-carbon transition will require meeting global energy demands with renewable sources of energy. Due to the intermittent nature of many renewable sources, achieving significant levels of integration will demand utility-scale energy storage systems. Li-ion batteries have dominated the market. However, rapidly growing demands in many technology sectors (e.g. electric vehicles, mobile electronics) aggravates the supply chain issues of critical elements, especially lithium, cobalt, nickel. This presents an urgent need of developing battery electrode materials with earth abundant elements (e.g. sodium, manganese) using sustainable technologies (e.g. dry-method).
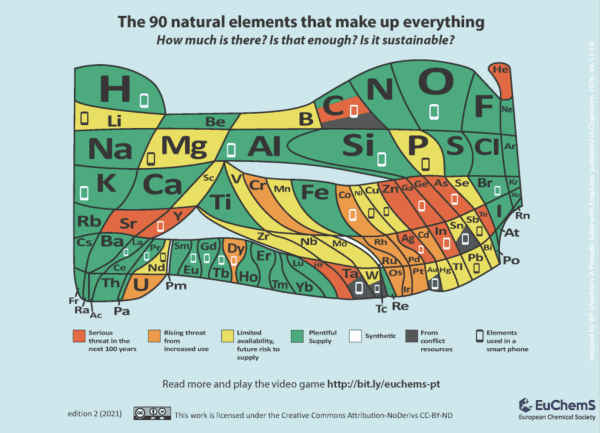
Image credit: EuChemS/CC BY-ND
Atomistic Mechanisms Analysis
Residential Faculty
Xiang Chen and Qiang Zhu